Tel: +86-18874804206 E-mail: sales@innovequipment.com
- All
- Product Name
- Product Keyword
- Product Model
- Product Summary
- Product Description
- Multi Field Search
- Home
- Products
- About Us
- News
- Support
- Application
- Contact Us
Views: 1637 Author: Site Editor Publish Time: 2024-09-01 Origin: Site
Blister packaging is an efficient, cost-effective, and protective solution for solid dosage forms. The manufacturing process involves five core steps: molding, filling, sealing, coding, and trimming. Each step requires precise control to ensure product quality and stability. Through  advanced online inspection technologies and rigorous quality control systems, blister packaging guarantees the safety and efficacy of pharmaceutical products throughout storage and usage.
advanced online inspection technologies and rigorous quality control systems, blister packaging guarantees the safety and efficacy of pharmaceutical products throughout storage and usage.
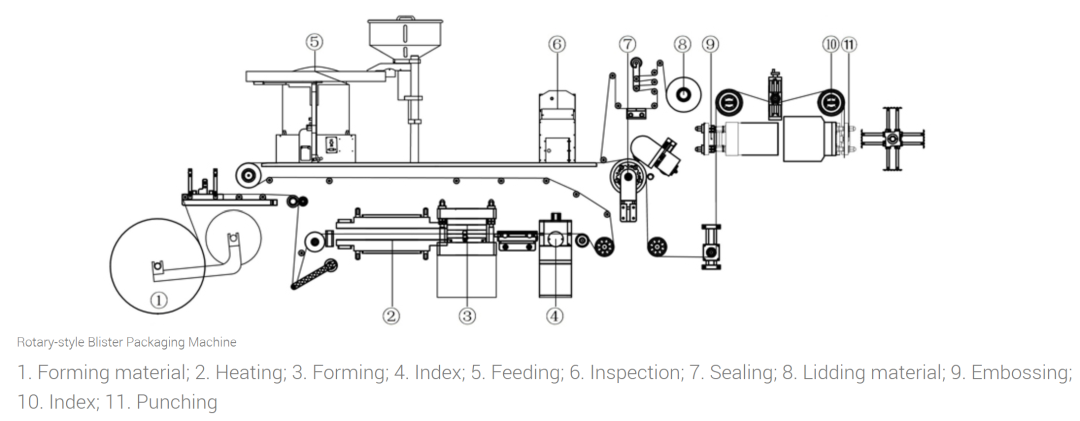
The first step in blister packaging is creating the drug containment cavity, known as the blister. On the production line, molding materials such as PVC, PVDC-coated PVC, or cold-formed aluminum are fed into heating equipment. During thermoforming, the plastic film is heated to 110-140°C and then pressed through a mold under negative pressure to form regular grooves. For cold-formed aluminum, no heating is required – instead, it's directly shaped through high-pressure stamping. The quality of blister formation directly determines the packaging stability of pharmaceuticals, requiring precise control of temperature, molding pressure, and time to ensure uniform shape and accurate dimensions.
After molding completion, pharmaceutical products are precisely dispensed into blister packs. Tablets, capsules, or micro tablets are conveyed by automated feeding systems and inspected through sensors or camera detection systems to ensure complete filling of each blister pack. The system automatically removes any defective items detected—such as missing tablets, overlapping layers, or damaged components. To maintain product integrity, the filling process must prevent dust contamination while controlling static electricity to avoid drug adhesion on conveyor belts that could compromise dispensing accuracy.
After filling, the sealing process immediately begins. The closure material, typically composed of aluminum foil or composite film, features a specialized heat-sealing coating. When the aluminum foil and blister material enter the heat-sealing zone, they bond securely under 180-250°C temperature and appropriate pressure, forming a sealed package. Seal integrity is critical: a loose seal may allow moisture ingress, while an overly tight seal could damage the blister structure. Therefore, temperature, pressure, and sealing time must be strictly controlled, with seal integrity verified through peel tests and airtightness assessments.
The sealed blister packaging must display the drug's batch number, production date, and expiration date for quality traceability. Common coding methods include inkjet printing and embossing. Inkjet printing requires ink that dries quickly and resists smudging, while embossing uses molds to press characters, ensuring clarity. The coding process must maintain legible text without blurring or missing details, guaranteeing fully readable information.
The final step involves packaging the completed blister packs into the cutting process. Cutting tools are used to slice blister panels into individual or multiple packaging units according to preset dimensions, accommodating various package specifications. The cutting precision must be maintained within ±0.2mm to ensure uniform size consistency across all units, facilitating subsequent carton assembly and distribution. Excessive cutting errors could compromise the compatibility of automated packaging equipment and even result in defective products.


Thermoforming is the primary process for blister packaging. By heating plastic films (such as PVC or PET) to a softened state, the material is then shaped under pressure or vacuum to create product-sized grooves. This technique is ideal for small items like tablets, offering high production efficiency, excellent packaging transparency, and enhanced product presentation.
Cold forming is primarily used for highly sensitive products (such as pharmaceuticals), employing aluminum foil composite film that is directly pressed into shape in molds, providing excellent moisture-proof, light-shielding, and oxygen-barrier properties. However, due to the opaque nature of cold-formed blister packs and their large space requirements, which affect packaging compactness, they are mainly used in applications requiring extremely high protection performance.
This innovative process combines the transparency of thermoforming with the superior barrier properties of cold forming. By first creating a clear layer through thermoforming, followed by an additional cold-formed layer, it delivers top-tier protection. This method is widely used in high-value, sensitive pharmaceutical products, effectively balancing visual clarity with long-term storage requirements.

The whole blister packaging process requires strict compliance with GMP for quality monitoring. Key parameters such as blister forming quality, drug filling, closure integrity and cutting accuracy should be detected and recorded online. Common detection methods include:
Visual inspection: used to check blister integrity, drug loss or foreign body contamination.
Leak detection: Use bubble method or vacuum attenuation method to ensure the sealing of the seal.
Peel test: verify the sealing strength to ensure that the packaging is firm and durable.
In addition, stability testing is required to simulate the storage of drugs in high temperature, high humidity or low temperature conditions to ensure that the packaging material provides adequate protection.
content is empty!