Tel: +86-18874804206 E-mail: sales@innovequipment.com
- All
- Product Name
- Product Keyword
- Product Model
- Product Summary
- Product Description
- Multi Field Search
- Home
- Products
- About Us
- News
- Support
- Application
- Contact Us
Views: 743 Author: Site Editor Publish Time: 2024-02-20 Origin: Site
Dry granulation is a widely used dry granulation technology in the pharmaceutical industry, primarily employed to form coarse particles from powder mixtures before tablet compression or capsule filling. This technique proves particularly effective for materials with poor flowability or when significant particle size variations exist among formulation components, effectively preventing material segregation.

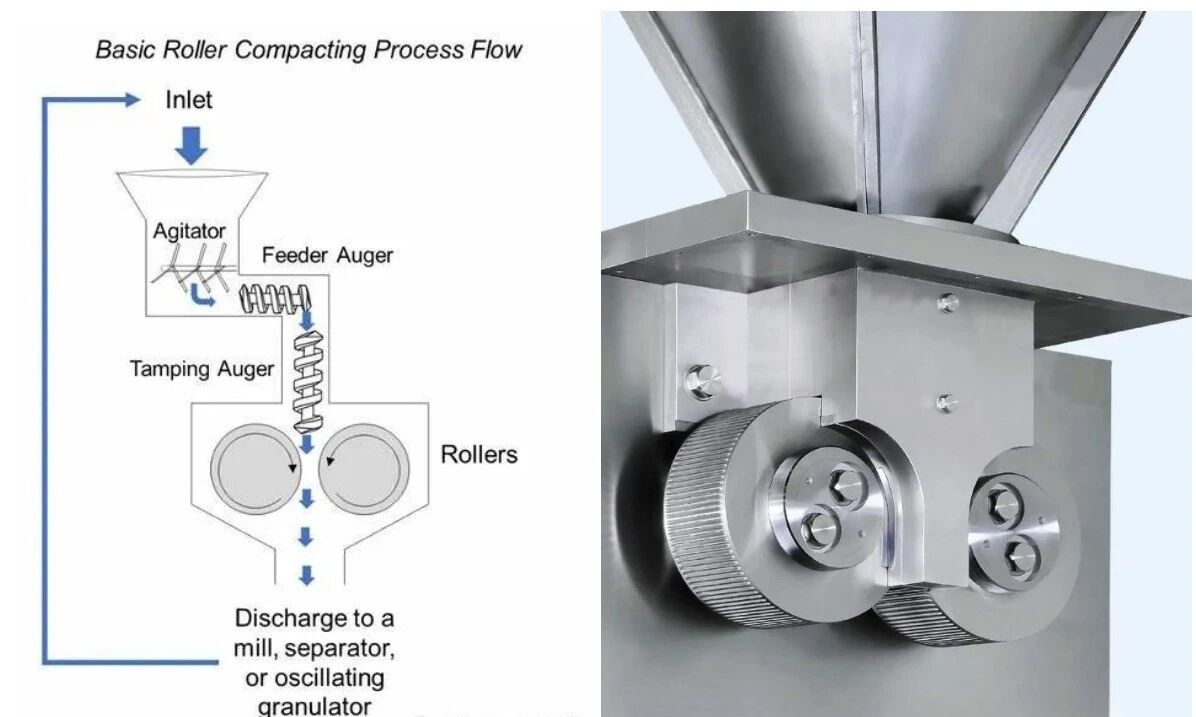
The basic principle of dry granulation
During the dry granulation process, the powder mixture enters the gap between two counter-rotating rollers through a hopper. The rollers compress the powder into dense ribbons, which are then ground into granules of the desired size. These granules exhibit excellent flowability, ensuring smooth and uniform distribution into tablet presses or capsule fillers. In this process, several critical process parameters significantly influence the final product quality:
1. Feed Rate
The feeding rate refers to the speed at which powder enters the nip zone between rollers from the hopper. Excessive feeding rates may cause blockages in the compactor, while insufficient feeding rates can lead to uneven thickness of sheet material and quality degradation. Therefore, optimizing the feeding rate is crucial to ensure an appropriate amount of mixture enters the compaction zone.
2. Compaction Pressure
Compaction pressure is determined by the gap between the rollers. Too much or too little pressure will cause the sheet to fail or become too fragile and break; too little or too much pressure will cause the compactor to stall and reduce process efficiency.
3. Roll Speed
Roller speed, along with other parameters, determines the retention time of the mixture in the compaction zone. Optimizing roller speed ensures high-quality and consistent sheet production. These three parameters (feed rate, compaction pressure, and roller speed) require coordinated optimization to ensure process efficiency and produce high-quality sheets suitable for subsequent processing.
Within the Quality by Design (QbD) framework, experimental design enables the establishment of acceptable ranges for each parameter. Poor control during dry granulation may compromise critical quality attributes (such as tablet hardness, tablet/capsule weight, tablet friability, or dissolution rate). Inadequate control could lead to excessive fine powder generation during grinding of compressed tablets, impairing flowability and resulting in weight inconsistencies or even content uniformity issues. The density and porosity of tablets not only affect downstream processing but also directly influence final product quality attributes, particularly dissolution rates. When implementing Process Analytical Technology (PAT), online density measurement ensures consistency in dry granulation unit operations, reducing downstream waste and product failures. Common measurement methods include skeletal density (Skeletal Density) measured via gas displacement. After weighing the sample, it is placed in a sample cup of known volume, filled with inert gas, and the volume is calculated by measuring gas pressure changes to determine skeletal density.


Envelope density is measured using specialized instruments. The process involves placing sheet materials into a highly fluid quasi-fluid medium, where displacement measurements are used to calculate the envelope density. Combined with skeleton density and sample mass data, this enables accurate pore fraction determination. Through real-time monitoring of material density and porosity, scientists and manufacturing support teams can more confidently ensure product consistency and quality. Variations in these parameters (both within and between batches) may indicate potential issues with raw materials or process parameters. Within the Process Automation Technology (PAT) framework, this online monitoring system helps detect deviations in production processes early, allowing for timely corrective actions to prevent defective products and save time and costs.
Dry granulation plays a vital role in pharmaceutical manufacturing, particularly when processing materials with poor flowability. By optimizing critical parameters such as feed rate, compaction pressure, and roller speed, combined with the quality by design philosophy, high-quality tablet products can be ensured, thereby guaranteeing the final product's quality. Through online density and porosity measurements, process design scientists can monitor and adjust the process in real time, ensuring product consistency and process efficiency.
content is empty!